The Biology Project > Biochemistry > The Chemistry of Amino Acids
Aspartic Acid D (Asp)
Chemical Properties: Physical Properties:
Acidic
Polar (charged)
Aspartic acid is one of two acidic amino acids. Aspartic acid and glutamic acid play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins.
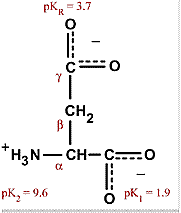
Proteins in the serum are critical to maintaining the pH balance in the body; it is largely the charged amino acids that are involved in the buffering properties of proteins. Aspartic acid is alanine with one of the β hydrogens replaced by a carboxylic acid group. The pKa of the β carboxyl group of aspartic acid in a polypeptide is about 4.0
Note that aspartic acid has an α-keto homolog, oxaloacetate, just as pyruvate is the α-keto homolog of alanine. Aspartic acid and oxaloacetate are interconvertable by a simple transamination reaction, just as alanine and pyruvate are interconvertible.
Oxaloacetate is one of the intermediates of the Krebs cycle.
Aspartic acid and oxaloacetate are interconvertable by a simple transamination reaction
The Biology Project > Biochemistry > The Chemistry of Amino Acids
http://www.biology.arizona.edu
All contents copyright © 2003. All rights reserved.