Energy, Enzymes, and Catalysis Problem Set
Problem 6: Equilibrium constant for ionization of acetic acid.
Correct!
The equilibrium constant for the ionization of acetic acid,
is 0.00002. What can you conclude about this reaction? |
A. It is a closed system. B. At equilibrium, the concentration of CH3COOH is much higher than the concentrations of CH3COO- + H+. C. It never reaches equilibrium. D. It is spontaneous, starting with CH3COOH. E. The equilibrium constant increases when the starting concentration of CH3COOH is increased.
Department of Biochemistry and Molecular Biophysics
University of Arizona
Wednesday, September 25, 1996
Contact the Development Team
http://www.biology.arizona.edu
All contents copyright © 1996. All rights reserved.
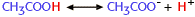 ,
,