Chapter 3
Initial Value Problems
3.2 An Initial Value Problem: A Cooling Body
Problems
- Find three solutions of each of the following differential equations.
- `(dy)/(dt)=-7y`
- `(dy)/(dt)=-7t`
- `(dy)/(dt)=-1.07y`
- `(dy)/(dt)=-1.07t`
- For each of the following differential equations, sketch the graphs of three solutions. (You may use your computer, your graphing calculator, and/or your solutions to Problem 1.)
- `(dy)/(dt)=-7y`
- `(dy)/(dt)=-7t`
- `(dy)/(dt)=-1.07y`
- `(dy)/(dt)=-1.07t`
- Find the solution of each of the following initial value problems.
- `(dy)/(dt)=-7y` with `y=20` at `t=0`
- `(dy)/(dt)=-7t` with `y=20` at `t=0`
- `(dy)/(dt)=-1.07y` with `y=2.3` at `t=0`
- `(dy)/(dt)=-1.07t` with `y=2.3` at `t=0`
- `(dy)/(dt)=-7(y-10)` with `y=20` at `t=0`
- `(dy)/(dt)=-7(t-10)` with `y=20` at `t=0`
- `(dy)/(dt)=-1.07(y-10)` with `y=2.3` at `t=0`
- `(dy)/(dt)=-1.07(t-10)` with `y=2.3` at `t=0`
- For each of the following initial value problems, sketch the graph of the solution. (You may use your computer, your graphing calculator, and/or your solutions to Problem 3.)
- `(dy)/(dt)=-7y` with `y=20` at `t=0`
- `(dy)/(dt)=-7t` with `y=20` at `t=0`
- `(dy)/(dt)=-1.07y` with `y=2.3` at `t=0`
- `(dy)/(dt)=-1.07t` with `y=2.3` at `t=0`
- `(dy)/(dt)=-7(y-10)` with `y=20` at `t=0`
- `(dy)/(dt)=-7(t-10)` with `y=20` at `t=0`
- `(dy)/(dt)=-1.07(y-10)` with `y=2.3` at `t=0`
- `(dy)/(dt)=-1.07(t-10)` with `y=2.3` at `t=0`
-
In the solution of the cooling body problem, we found that `k` is `ln 9 - ln 7`.
- Show that `-k=ln(7/9)`.
- Show that the solution function also can be expressed as
- Recall where the numbers in this expression came from: `21` is the room temperature, `9` is the difference between body temperature at `t=0` and room temperature, and `7` is the difference between body temperature at `t=1` and room temperature. Interpret what this new formula for `T` says about change in the first hour.
- Interpret the new formula for `T` as saying that the “same thing” happens in every hour. What same thing?
`T=21+9(7/9)^t`.
- You may think it unnatural to talk about room temperature and body temperature in degrees Celsius. Convert the room temperature, 21°C, and the body temperature at time of death, 37°C, to degrees Fahrenheit. Do these seem like reasonable numbers?
-
-
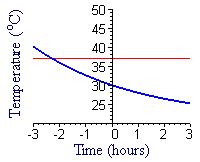
In Figure E1 we show a graph of `T=21+9e^(-kt)`, with `k=0.2513144`, along with a graph of normal body temperature, 37°C. Sketch your own graph (or click on the figure to get a printable copy of this one, if you prefer) of the industrialist's body temperature before and after death.
-
The graph of `T=21+9e^(-kt)` approaches a horizontal asymptote for large values of `t`. Extend your graph from part a to show this asymptote and the approaching graph.
-
What is the physical meaning of the approach of the temperature graph to this asymptote?
-
- In this problem we explore the possible complications created by setting `t=0` at time of death instead of at the time of first temperature measurement. We can still write the differential equation as
`(dT)/(dt)=-k(T-21)`,
and we can still transform the equation to`(dy)/(dt)=-ky`.
Thus the solution for `y` still has the form`y=y_0e^(rt)`
where `r=-k`.
- What should `y_0` be in this case?
- Suppose we call the time of first measurement `t_1` so that the time of second measurement is `t_1+1`. Suppose the body temperatures at these two times are, as before, 30 and 28°C, respectively. In light of our previous calculation, what do you expect `t_1` to turn out to be? What do you expect `k` to turn out to be?
- Use the solution for `y` and the two temperature measurements to write two equations in the two unknown quantities, `k` and `t_1`. Solve these simultaneous equations. Do you get the answers you expected?
- A company is considering two ways to depreciate a piece of capital equipment that originally cost `\$`14,000 and is worth `\$`10,000 after one year:
Method I assumes the equipment depreciates at a rate proportional to the difference between its value and its scrap value of `\$`400.
Method II assumes the equipment depreciates linearly, that is, at a constant rate.
- Using method I, find the value of the equipment at the end of 2 years and at the end of 3 years.
- Using method II, find the value of the equipment at the end of 2 years and at the end of 3 years.
- Which method produces faster depreciation? Explain.
-
 Radioactive substances tend to decay at a rate proportional to the amount present at any given time. Let `y_0` be the amount of a radioactive substance present at time `t=0`, and let `y=ytext[(]t text[)]` be the amount present at any time `t`.
Radioactive substances tend to decay at a rate proportional to the amount present at any given time. Let `y_0` be the amount of a radioactive substance present at time `t=0`, and let `y=ytext[(]t text[)]` be the amount present at any time `t`.
- Write an initial value problem whose solution is `ytext[(]t text[)]`.
- Solve your initial value problem to find a formula for `ytext[(]t text[)]`.
- The half-life of a radioactive substance is the time it takes for a given amount to decay to half that amount. Find an expression for half-life that depends only on the proportionality constant in your differential equation.
-
 (See Problem 10.) Superman has a violent reaction to green kryptonite, which fortunately has a half-life of only 15 hours as it decays into red kryptonite. It is no longer dangerous to Superman when 90% of the green kryptonite has decayed. If Superman is exposed to pure green kryptonite, for how long is he in danger?
(See Problem 10.) Superman has a violent reaction to green kryptonite, which fortunately has a half-life of only 15 hours as it decays into red kryptonite. It is no longer dangerous to Superman when 90% of the green kryptonite has decayed. If Superman is exposed to pure green kryptonite, for how long is he in danger? - (See Problem 10.)
-
What is the relation between the half-life `H` of a radioactive substance and the “third-life” `T` of the same substance?
-
What is the relation between the doubling time `D` of a colony of rabbits and the tripling time `T` of the same colony?
-
Problems 11 and 12 are adapted from Calculus Problems for a New Century, edited by Robert Fraga, MAA Notes Number 28, 1993.